Harpoon™ Percutaneous Fixation and Fusion Device
Manufactured by Implants International Limited
Product Buying Options
Additional Sales Information: Manufactured in Britain by Implants International Ltd and marketed through Xtremity Solutions Ltd.
Product Description
Suitable for a miscellany of indications including, but not limited to:
- Hammer Toes
- Lisfranc Fractures
- Claw Toes
- Midfoot Arthrodesis
- Mallet Toes
- Forefoot Arthrodesis
- Metatarsal Fractures
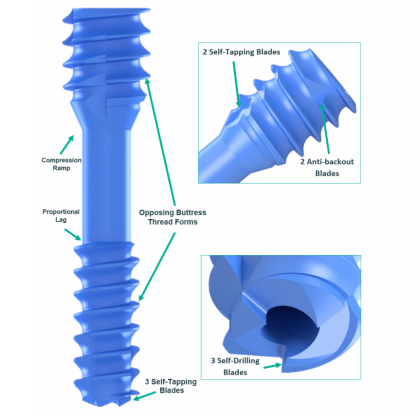
- No pre-drilling required
- Dual trocar ended: suitable for retrograde entry, followed by antegrade advance, placement and compression
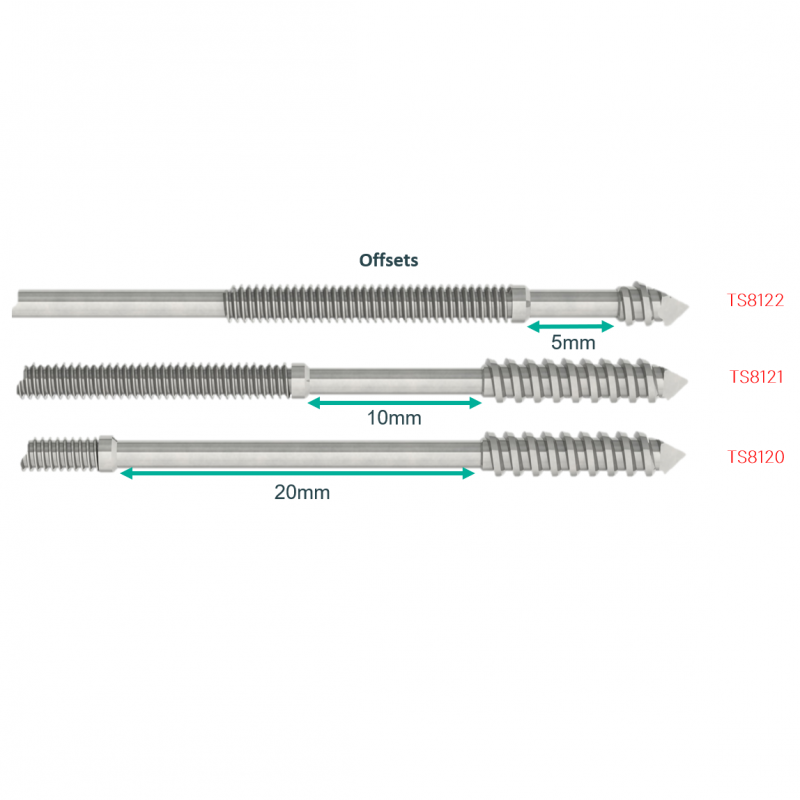
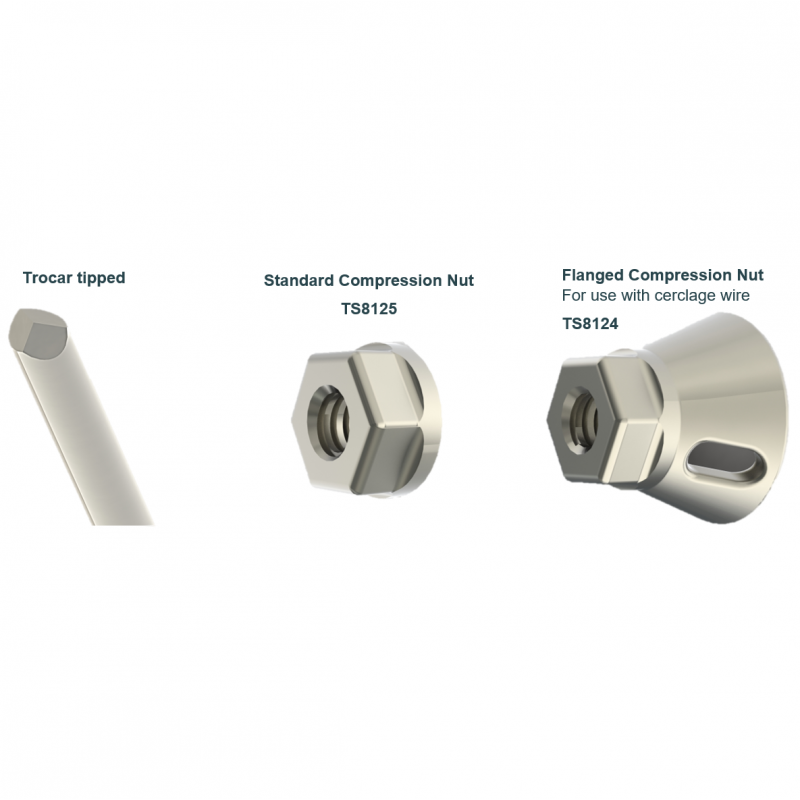
- Optimal fracture site compression with a choice of compression fittings
- Non-theatre retrieval of device